Global Standard Management System Certification

The ISO 9001 Quality Management System is an international certification system established and implemented by the International Organization for Standardization (ISO). It provides objective evaluations by third-party certification bodies regarding the fulfillment of requirements in terms of minimizing supply chain risks and conditions for the products and services supplied to customers. This system offers credibility to both buyers and suppliers.
By improving the overall quality level of enterprises, it ensures competitive advantages and enhances Client satisfaction, thereby boosting the company’s competitiveness. This system aims for the long-term growth and development of enterprises.
Quality Management System
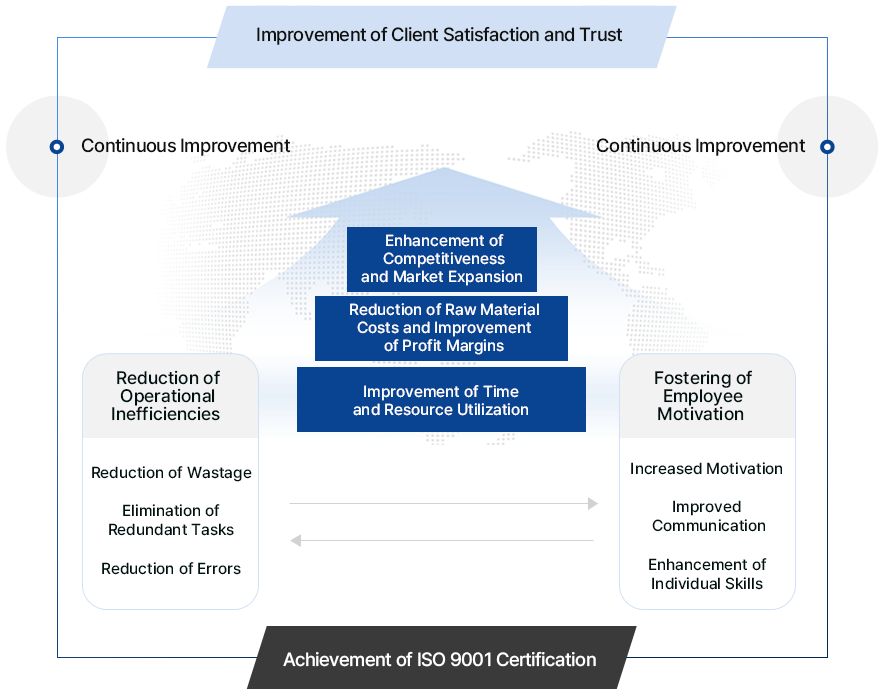
Establishment of an appropriate quality management system and management procedures in accordance with international standards
Continuous improvement through the standardization and documentation of quality manuals and other processes
Elimination of irregularities within the company
Facilitation of international trade transactions
Assurance of benefits by eliminating defective products and quality issues
Assurance of quality competitiveness through systematic cooperation
Efficient use of management resources
Enhancement of trust and marketing capabilities regarding Clients
Improvement and internalization of quality awareness
Assurance of quality competitiveness through consistent and reliable operations
Enhancement of corporate image
Response to Product Liability (PL)
Competitive advantage in government procurement
Smooth inspection and assessment during buyer factory audits (priority given to certified companies)

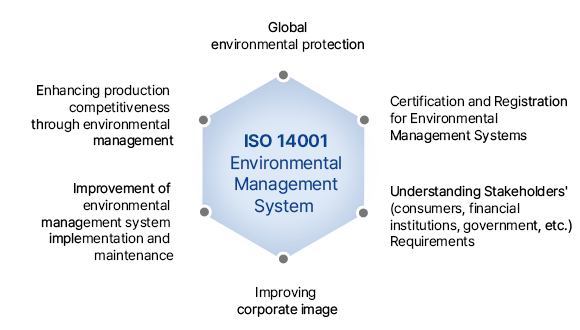
The ISO 14001 standard defines a set of management activities aimed at improving the sustainable environmental performance of an organization throughout all stages of its operations. This international standard, established by the International Organization for Standardization (ISO), pertains to environmental management systems. The certification system assesses and objectively certifies whether an organization's environmental management system complies with this standard by a third-party certification body.
ISO 14001, unlike various international regulations, evaluates and certifies the comprehensive environmental management system applied across all corporate activities. It goes beyond mere compliance with environmental laws or international standards, assessing how effectively a company conducts comprehensive environmental management, including environmental audits, planning, implementation, operation, monitoring, corrective actions, management review, and continuous improvement.
Environmental
Management
System
Improvement of corporate image as an environmentally friendly company
Reduction of major environmental risks and enhancement of customer reliability
Reduction in the likelihood of violating environmental laws/regulations
Strengthened relationships and responses with stakeholders (government, investors, insurers,
creditors, etc.)
Reduction in waste treatment costs
Savings in raw materials and energy
Reduction of costs through environmental, safety, and zero-accident initiatives
Enhanced ability to predict and respond to laws and regulations for business sustainability
Customer acquisition through environmental improvement
Improvement of environmental performance and environmental health
Continuous reduction and management of environmental impacts
Expansion of market competitiveness


Entering the 21st century, as the global economy accelerates and knowledge-based industries grow, various technologies and management innovations are being strategically deployed worldwide. In line with this global trend, international standardization activities in management systems are also being fundamentally established. In response to these international movements, the International Organization for Standardization (ISO) established and announced the international standard for food safety management, ISO 22000, in September 2005.
ISO 22000 is an international standard that specifies requirements for food safety. It defines a food safety management system that encompasses all elements needed to control food safety hazards throughout the food supply chain. This standard aims to enhance food safety by systematically managing all organizations involved in the food supply chain.

It is Necessary to Secure Food Safety and Secure and Maintain International Reliability.

ISO 45001 (Occupational Health and Safety Assessment Series) is an occupational health and safety management system. Unlike previous safety activities focused mainly on safety managers, ISO 45001 requires the active participation of the highest management and all members of the organization. This proactive participation aims to predict and prevent risks that may occur in actual activities, ensuring the safety and health of employees and systematically managing the organization's safety.
ISO 45001 is not merely a standard for auditing and guiding occupational health and safety management systems; it is a system that enhances the performance of industrial conditions and safety activities.
Furthermore, ISO 45001 is designed to be integrated with the systems of ISO 9001 and ISO 14001, providing a unified direction for management.
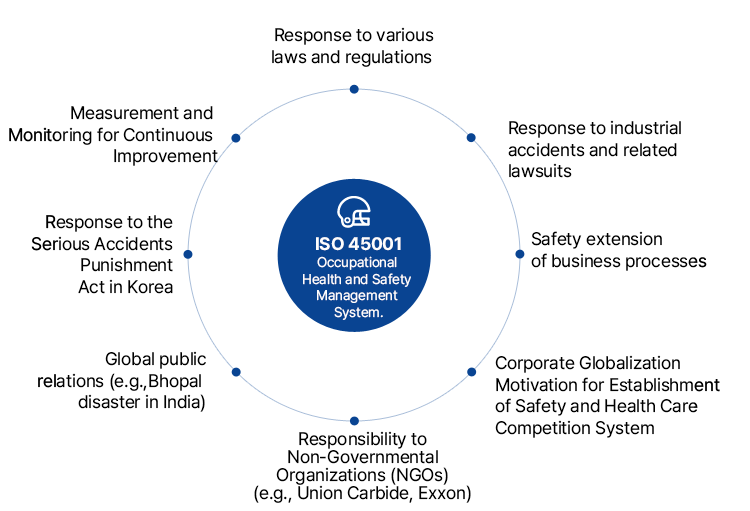
Establishment and continuous improvement of a systematic occupational health and safety management system at the workplace
Promotion of effective occupational health and safety management through competitive evaluation
of corporate risks
Enhancement of public trust and social image regarding stakeholders
Contribution to the reduction of accidents, occupational diseases, and other liabilities, improving productivity and employee welfare
Increase in export competitiveness by overcoming trade barriers in the safety sector
Reduction of non-conformities due to workplace environment improvements
Possibility of optimal integration with ISO 9001
and ISO 14001
Contribution to labor-management stability through participation in the overall safety management system

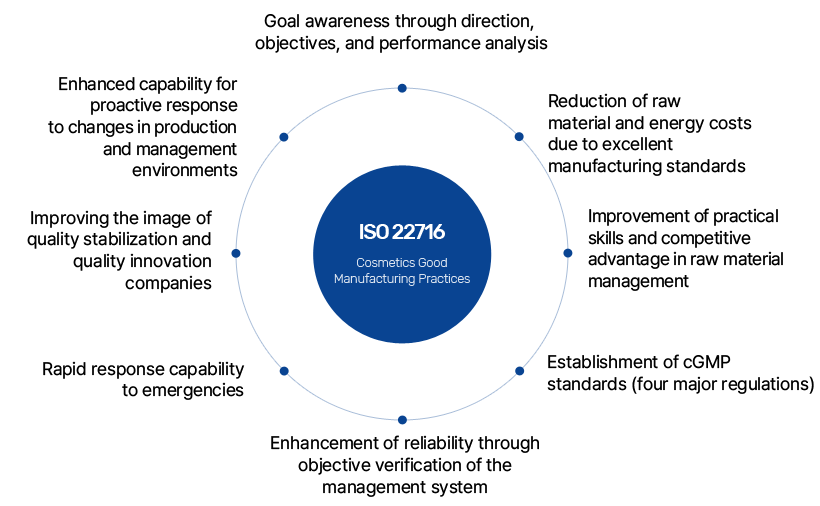
ISO 22716 provides guidelines for the production, control, storage, and shipment of cosmetic products to ensure their quality. However, it does not apply to aspects related to the safety and environmental protection of employees working in the factory. These safety and environmental aspects are the company's responsibility and must comply with current laws and regulations. Additionally, this guideline does not apply to research and development activities or the distribution of finished products.
ISO 22716 is currently offered as a self-certification service.
Creams, emulsions, lotions, gels, and oils for the skin (hands, face, feet, etc.)
Face masks
Foundation bases (liquid, paste, powder)
Makeup powders, after-bath powders,
hygiene powders, etc.
Perfumes
Bath and shower preparations
Deodorants (antiperspirants), sweat inhibitors
Hair care products
Shaving products (creams, foams, lotions, etc.)
Makeup and makeup removers (face, eyes, lips)
Dental and oral care products
Nail care and makeup products
Anti-wrinkle products
Skin whitening products
Suntan and sun care products
Others
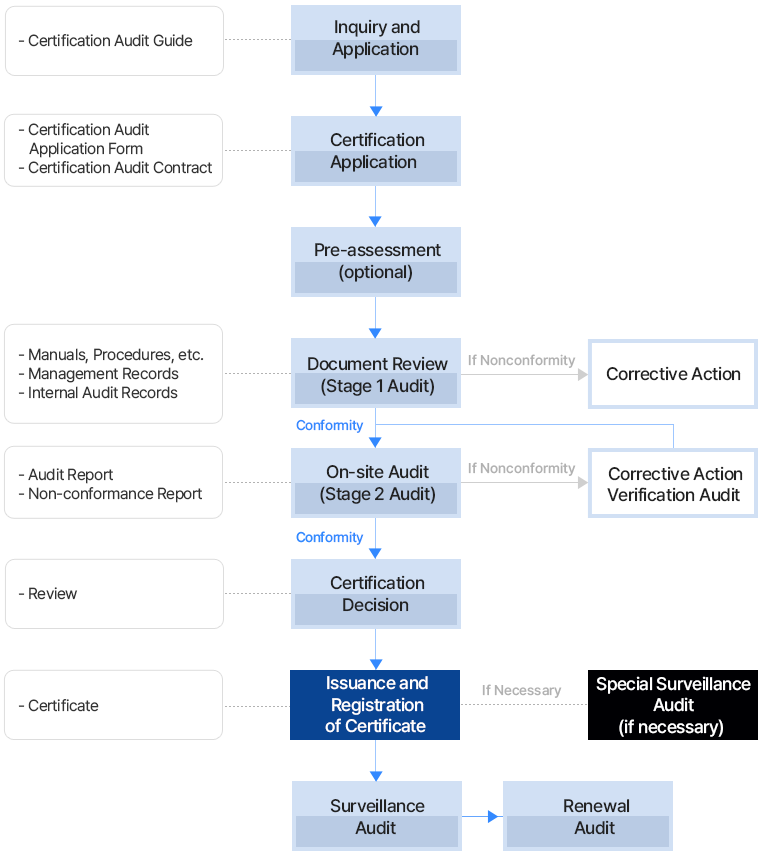
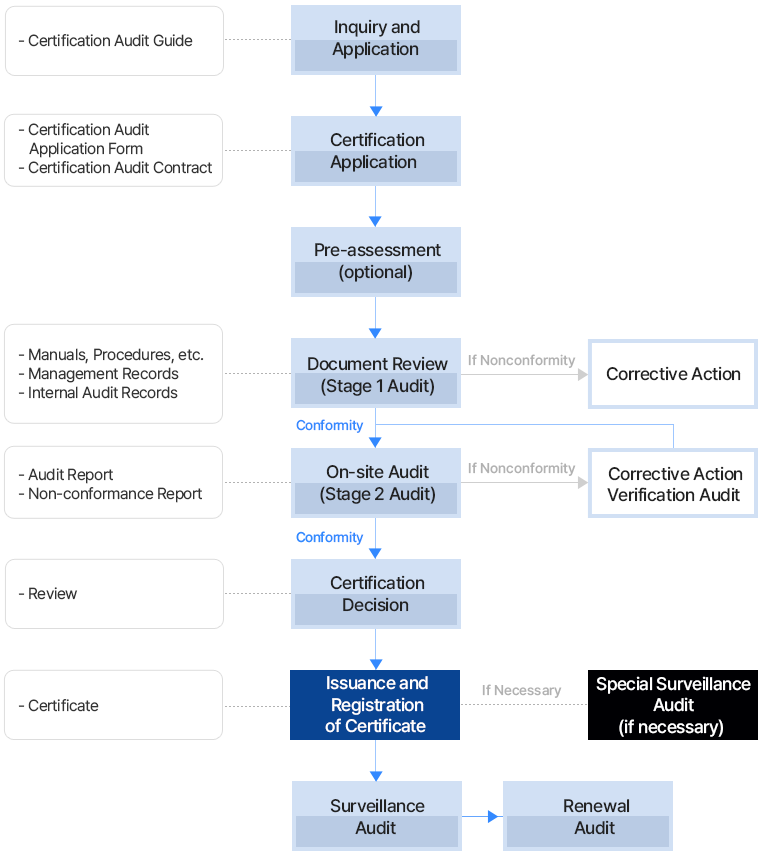
Audit days (MD) refer to 8-hour workdays, including time for breaks, meals, and preparation/administrative tasks for the audit (up to 20% of the total time).
| Number of Employees | Initial Audit (Stage 1 and 2) | Surveillance Audit (Stage 2) | Recertification Audit (Stage 1, 2, or Stage 2) |
|---|---|---|---|
| 01 ~ 20 | 3 | 2 | 2 |
| 21 ~ 50 | 4 | 2 | 3 |
| 51 ~ 100 | 5 | 2 | 3 |
| 101 ~ 300 | 6 | 2 | 4 |
| 301 ~ 1,000 | 7 | 3 | 4 |
| 1,001 ~2,000 | 8 | 3 | 5 |
| 2,001 ~4,000 | 10 | 3 | 6 |
| Over 4,000 | - | Increase as above | - |
*The number of employees in the company eligible for ISO 22716 certification audit is used as the basis (including part-time, temporary, and contract workers).
*The number of audit days may be adjusted based on the certification process submitted by the company when applying for certification (this applies at the time of data submission).
For example, if you submit documents such as process flowcharts, organizational charts, proof of employee numbers, or photos of products or services, employees not involved in the scope of certification will be excluded.

An ISMS (Information Security Management System) is a management system based on a systematic business risk approach to establish, implement, operate, monitor, review, maintain, and improve information security. ISO/IEC 27001 is the international standard that defines the requirements for an ISMS, allowing for the only auditable and certifiable standard for information security management systems. This standard is designed to ensure that companies can manage their valuable information assets securely and consistently through a comprehensive control framework. Failure to ensure information security can pose significant risks, not only to the company but also to the sensitive information of customers associated with the company.
The ISO 27001 certification involves a step-by-step process of thoroughly examining the organization's information assets and assessing the risks associated with these assets. Personnel involved in this process can analyze and monitor the likelihood of threats or breaches from external sources, the impact of these external threats or breaches on the organization, and the effectiveness of controls to protect the assets. This enhances the reliability and security of the system.

Increased business opportunities as customers
/suppliers recognize a reliable partner.
Independent verification of compliance with applicable laws and regulations.
Business differentiation factors providing a competitive edge over similar organizations.
Prevention of leakage of internal information assets
and personal data.
Enhanced monitoring and management controls.

ISO 13485:2016 specifies the requirements for a quality management system that an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. This includes organizations involved in one or more stages of the life cycle, including the design and development, production, storage and distribution, installation, servicing (e.g., technical support), and associated activities (e.g., technical support). The requirements of ISO 13485 are applicable to any organization regardless of its size and regardless of its type except where explicitly stated. ISO 13485 can also be used by suppliers or external parties providing products or services, including quality management system-related services to such organizations.
Outlines methods for reviewing and improving processes across the organization, increasing efficiency, reducing costs, and monitoring supply chain performance.
Demonstrates the production of safer and more effective medical devices.
Enhances global market access through certification.
Provides an overview of methods for reviewing and improving processes throughout the organization.
Improves efficiency, reduces costs, and monitors supply chain performance.
Demonstrates the production of safer and more effective medical devices.
Meets regulatory requirements and customer expectations.
| Certification Sectors | |
|---|---|
| Non-Active Medical Device Technical Area |
General Non-Active, Non-Implantable Medical Devices |
| Active Medical Devices (Non-Implantable) |
General Active Medical Devices |
| Parts and Services |
Raw Materials |
| In Vitro Diagnostic Medical Devices (IVD) |
- Reagents and reagent products, calibrators and control |